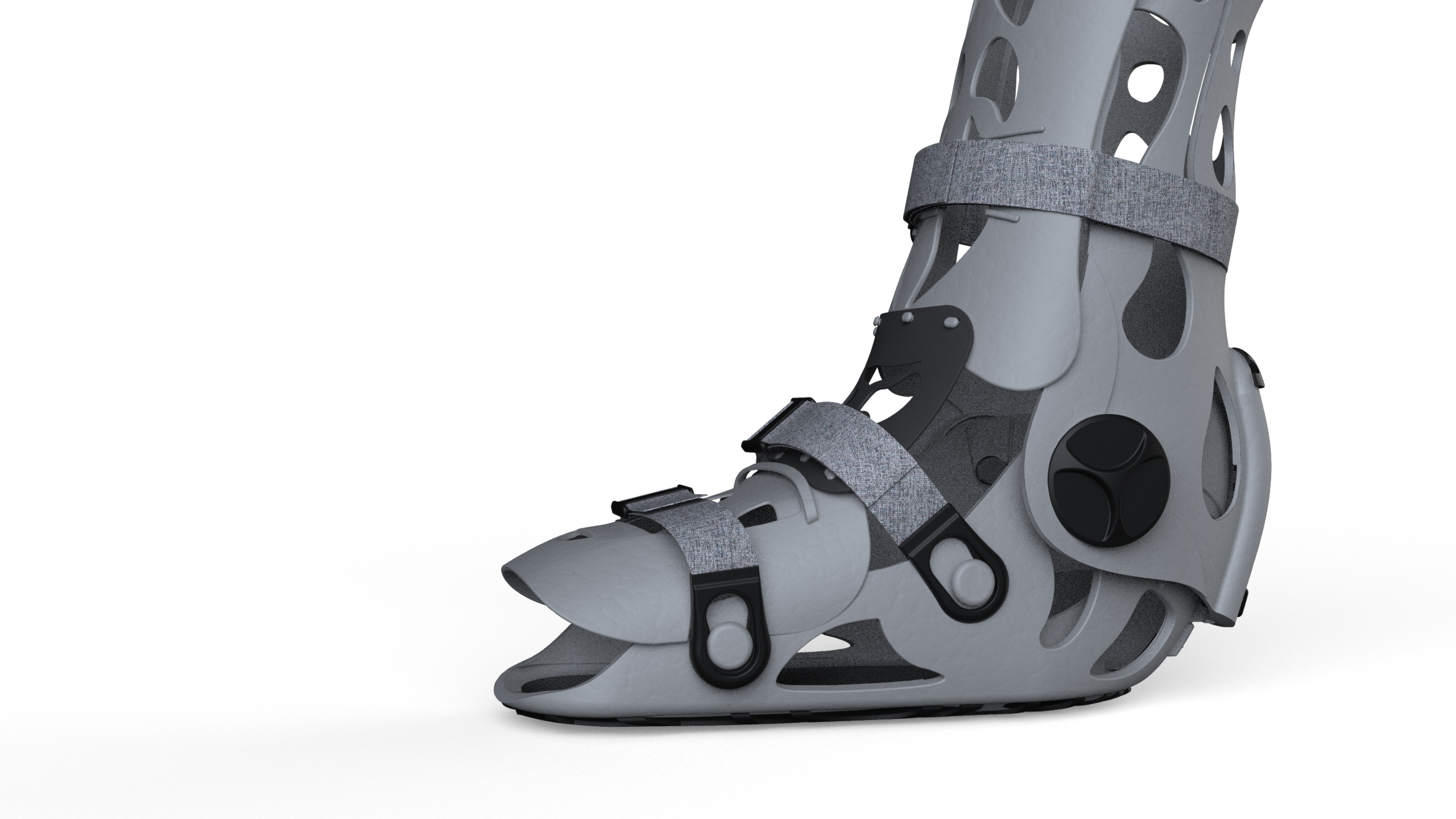
Where Precision Meets Care.
Medical products don’t just need to function they need to save lives, support clinicians, and meet some of the world’s most demanding standards. At MILIVOJA, we design medical devices with the gravity and clarity the field deserves. Our process bridges clinical reality with technical precision, ensuring that every concept moves forward with purpose, compliance, and care.
We help innovators navigate the complexity of healthcare product development, from napkin sketch to regulatory clearance. Whether you’re developing a surgical instrument, diagnostic wearable, or connected home device, we support you with the engineering, design, and regulatory foresight required to build something that truly improves patient outcomes.
More Than a Product. It’s a Promise.
Developing a medical device means building trust with doctors, patients, regulators, and your own team. That trust is earned by designing something that works under pressure, integrates seamlessly into clinical workflows, and holds up to the highest global standards.
Before we design anything, we define purpose, users, environments, and risks. We investigate clinical need, perform competitive analysis, and identify regulatory paths early. We don’t just make devices that can be built. We make devices that should be built.
Structured. Transparent. Compliant.
Medical device development is complex. But with the right framework, it doesn’t have to be chaotic. We offer a process that aligns technical design with regulatory reality and human need.
We start by aligning stakeholders and mapping the product vision. Who is the end user? What are their constraints? What clinical setting will this live in? We define core requirements, usability goals, and early risks. We consider ISO 14971 from the beginning. The goal is to establish a clear architecture that drives design and compliance in parallel.
We develop a clear, actionable roadmap. This includes resource timelines, risk mitigation strategies, regulatory documentation plans (510(k), CE Mark, etc.), and QMS alignment (ISO 13485). Every decision here sets up the rest of the program to succeed and scale, ensuring that innovation is matched with compliance and long-term sustainability.
With strategy in place, we dive into development. Our team produces detailed designs across mechanical, electronic, and embedded software components. Prototyping is fast, functional, and ongoing. We test usability, ergonomics, and robustness at every phase, building toward a manufacturable and reliable solution.
This is where we prove it works on paper and in practice. We perform bench testing, electrical safety (IEC 60601), mechanical durability, and environmental assessments. Our process ensures traceability from design input to test result. We document thoroughly, not because we have to, but because that’s how quality is built.
We validate with users in real or simulated settings. We support clinical evaluations and pre-submission packages. We finalize documentation (Design History File, Technical File) and prepare for regulatory review. Pilot production is launched, and design is locked.
These stages aren’t rigid. We adapt based on project size, maturity, and regulatory classification. What stays constant is our commitment to technical integrity, patient safety, and clear communication.
We Design with Intention. We Engineer with Integrity.
From concept sketch to pilot build, we do it under one roof. Industrial design, mechanical engineering, electronics, and rapid prototyping aligned, fast, and quality-driven.
We bake compliance into every phase. Whether it’s FDA QSR, MDR, ISO 13485, or IEC standards, we know what’s coming—and we design for it upfront. That saves time, money, and risk.
We collaborate with clinicians, observe in-use settings, and apply human factors engineering. We believe good devices must be intuitive to use and impossible to misuse.
Our workshop builds more than looks-like models. We test ideas with real-world function, so feedback is grounded in action. We iterate fast, and improve faster.
We measure success by patient impact. That’s why we don’t just build devices that meet spec. We build tools that improve care.
We share progress weekly. We track issues openly. We make decisions with you, not for you. Clients trust us because we communicate clearly and deliver consistently.
Let’s Build What Healthcare Needs
Medical device development is hard. But with the right team, it doesn’t have to feel impossible. If you’re building something that could make lives better, we’re here to help make it real.
